The Pharmaceutical Market Size accounted for USD 1.5 Trillion in 2022 and is projected to achieve a market size of USD 2.8 Trillion by 2032 growing at a CAGR of 6.4% from 2023 to 2032.
Active Pharmaceutical Ingredient (API) refers to drugs and medications used for the prevention, treatment, and diagnosis of diseases and medical conditions. APIs find application in high-quality drugs that treat diseases in oncology, cardiology, CNS and neurology, pulmonology, gastroenterology, nephrology, ophthalmology, and endocrinology.
The pharmaceutical industry plays a crucial role in healthcare by researching, developing, producing, and marketing these drugs.
Demands for pharmaceutical preparations are at an all-time high and there is an increasing need to abide by stringent quality regulations. To stay competitive, it is more important than ever to optimize process efficiencies in to save production costs and avoid the consequences of downtimes.
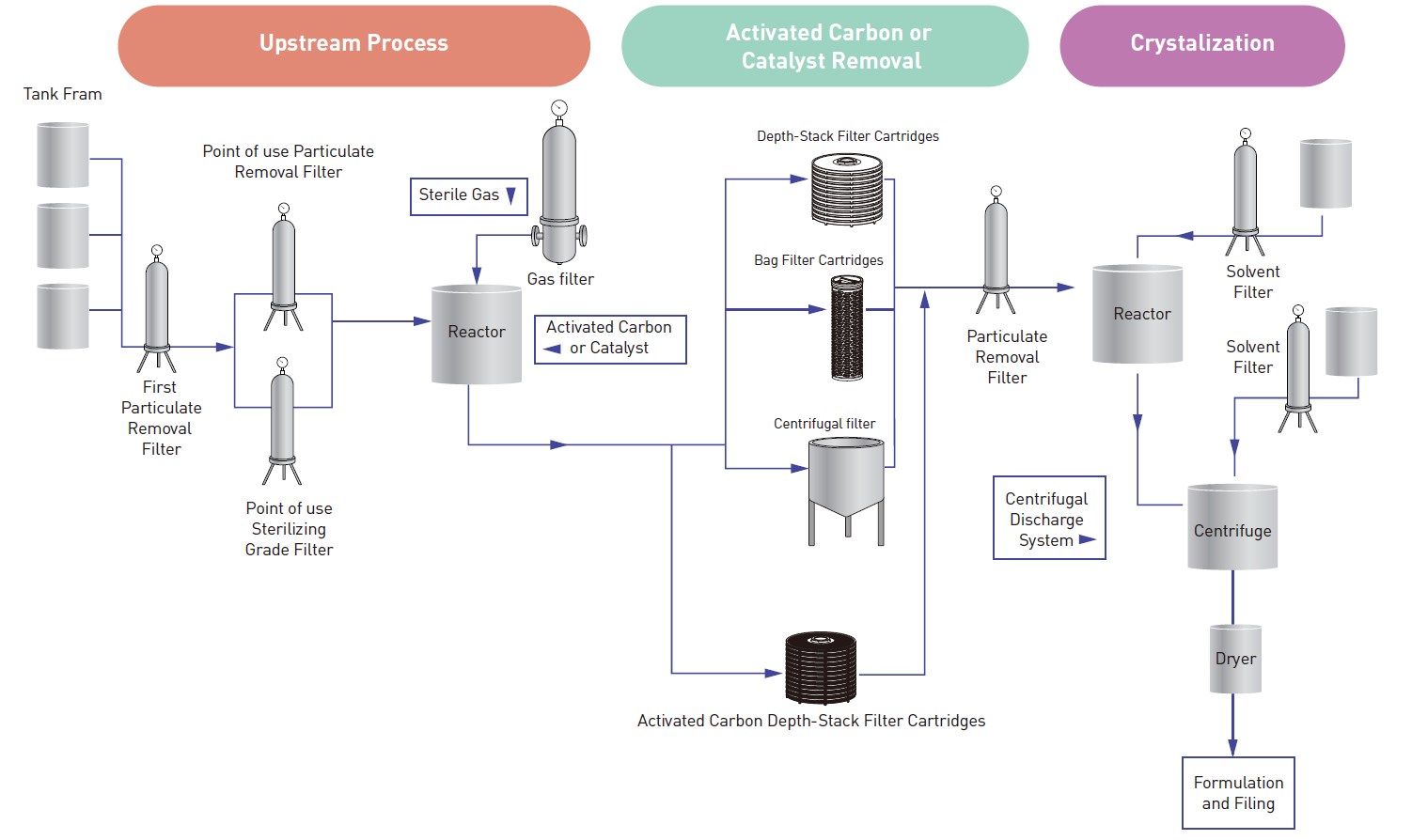
In the API, the process of filtration and separation is crucial. So the typical production process includes screening and filtering of the important active pharmaceutical ingredients and excipients procured use to eliminate foreign contaminants such as paper, grits, stone, threads, etc. and oversized particles & lumps to achieve the right drug formulation.
Pharmaceutical processing requires a high level of filtration to ensure the safety and efficacy of the drugs produced.
Active Pharmaceutical Ingredient (API)
Filtration of raw materials is important to ensure the best productivity during chemical reaction or treatment, ensure that the final product is pure and of high quality and remove contaminants from solids particles to microorganisms.
During production, it could be necessary to add solids such as active carbon or catalysis which will be removed after the treatment or reaction. Depending on the solid quantity a first main step could be necessary to remove the majority of the active carbon and catalysts and most of the solid impurities, and foam after fermentation.
In this step, Darlly recommends Filter Bag, Bag Filter Cartridges or Depth-Stack Filter Cartridges to remove most of the solid.
The Depth-Stack filter sheets are made from cellulose fibers and inorganic filter aids
(Diatomaceous earth etc.), it has three functions of surface filtration, depth filtration and electrostatic adsorption.
Sterile filtration is a technique used to remove or reduce microbial contaminants from liquids. Yeasts, molds, bacteria and other organisms can be found everywhere. Therefore, barriers such as filters should be installed to remove bacteria and other organisms.
Darlly's PES, PTFE or N66 cartridges in 0.45µ m/0.22µm ratings are used extensively in the pharmaceutical industry to provide secure and reliable removal of spoilage microorganisms in a variety of applications. To guardian the sterile cartridges, 1-3µm rating cartridges for
pre-filtration can be added if necessary.
These cartridges can be steamed in place (SIP) and hot water cleaned, without compromising their robustness or removal performance. Additionally, to confirm cartridge performance, an integrity test can be executed both prior to and after completion of each filtration batch.
All sterile filter cartridges have validated for the retention of B.diminuta (ATCC 19146) at a challenge level of 107 cfu per cm2 membrane.
It is very important to ensure the venting sterility which can be used in tanks of storage, preparation, fermentation, bright and incubation, etc., and the fermentation process.
In the Sterile compressed air process, Darlly recommends 0.3µm-0.5µm removal rating PP/GF cartridges as pre-filtration, 0.2µm (0.003µm for gas) absolute rating PTFE cartridges sterile filtration for sterile air/gas and 1µm Stainless steel powder sintered filter cartridges for cleaning steam and then sanitizing the cleaned equipment.
CIP is the removal of soil from product contact surfaces in their process position by circulating, spraying or flowing alkaline. Because the used CIP caustic solution contains mechanical impurities such as particles, suspended solids, microorganisms, resin, oil etc., so which needs to be filtered to facilitate water reuse and reduce wastewater discharge.
Filtration Solutions for Sterile API