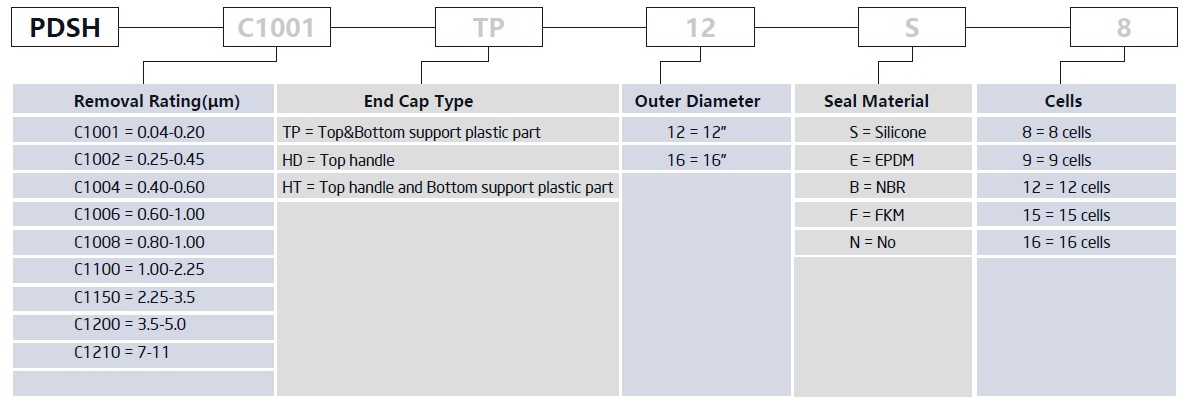
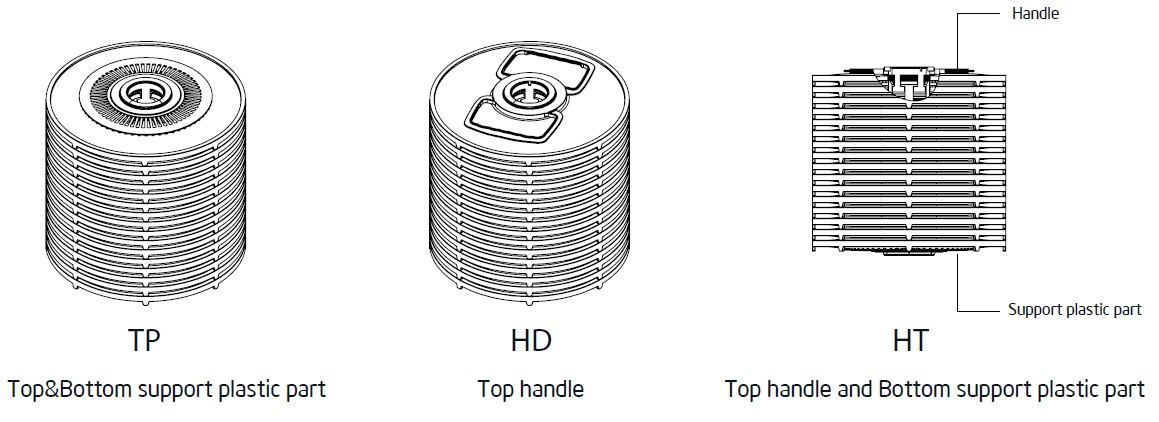
PureDisc™ Depth-Stack Filter Cartridges
PureDisc™ Depth-Stack Filter Cartridges are available in multi-material filter sheets, which are widely used in pharma-ceutical and food & beverage industries. The filter sheets are made from cellulose fibers and inorganic filt...
PureDisc™ Depth-Stack Filter Cartridges are available in multi-material filter sheets, which are widely used in pharma-ceutical and food & beverage industries. The filter sheets are made from cellulose fibers and inorganic filter aids (Diatoma-ceous earth etc., it has three functions of surface filtration, depth filtration and electrostatic adsorption.
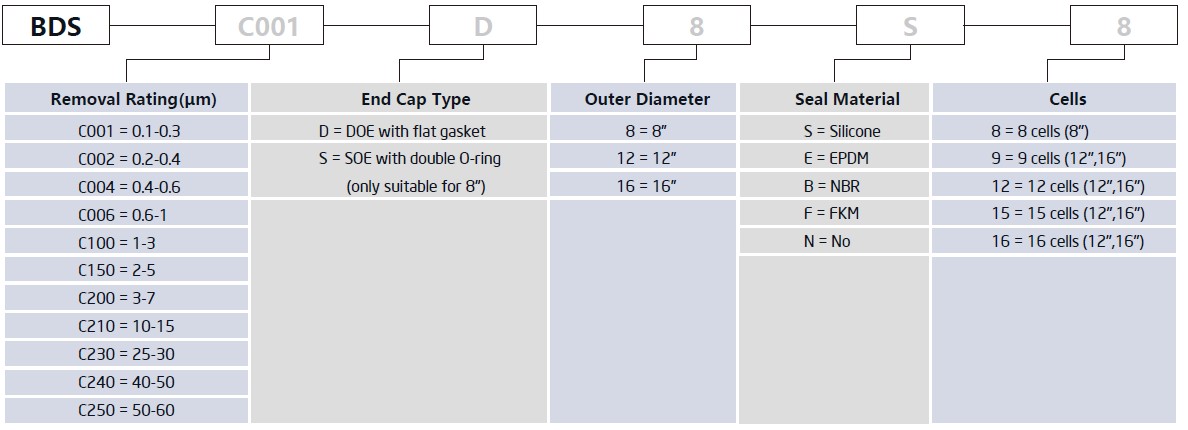
BDS series adopt domestic filter sheets, which shows excel-lent high throughout and cost-effective performance. General-ly, larger particles in feed liquids are mechanically intercepted through three-dimensional and porous structure, while smaller particles and microorganisms are intercepted by positively charged electrostatic adsorption.
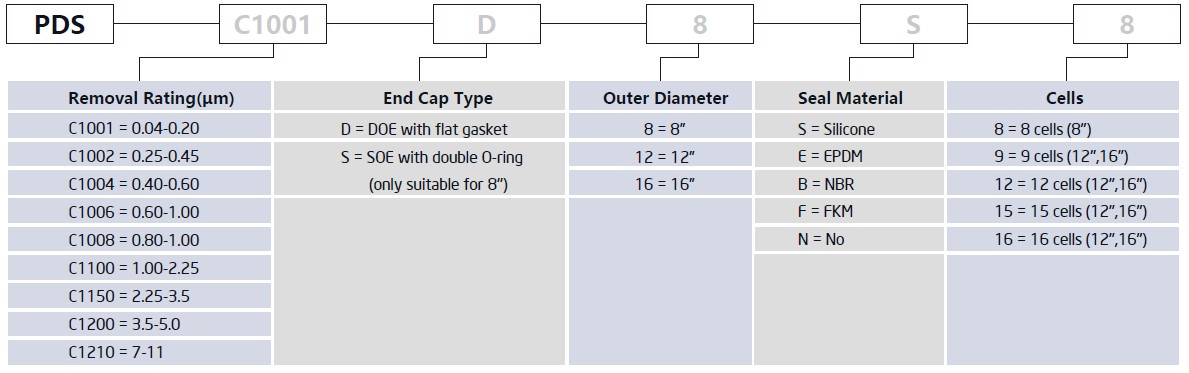
PDS series adopt imported filter sheets, which has excellent features as like low density, high porosity, large particle removal capacity and long service life etc. The product is mainly suitable for filtration of viscous materials, colloid particles or coarse dispersed materials with low differential pressure.
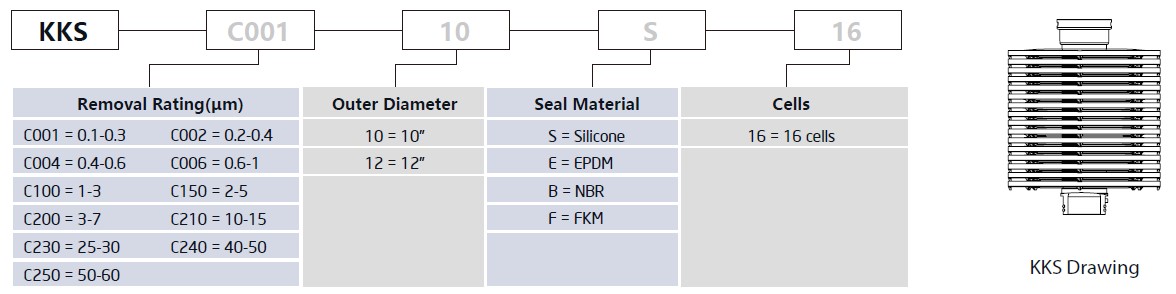
KKS series adopt domestic filter sheets, the unique buckling connection allows for stack-to-stack usage and it is easy to be installed and dismantled.
| Dimension | |
| Cells | 8 cells / 9 cells / 12 cells / 15 cells / 16 cells |
| Outer Diameter | 8", 10", 12", 16" |
| Filtration Area | 0.36m² (3.9ft2)(Φ8", 8cells) 1.44m² (15.5ft2)(Φ10", 16cells) 1.08m² (11.6ft2)(Φ12", 9cells) 1.44m² (15.5ft2)(Φ12", 12cells) 1.8m² (19.4ft2)(Φ12", 15cells) 1.92m² (20.7ft2)(Φ12", 16cells) 2.34m² (25.2ft2)(Φ16", 9cells) 3.12m² (33.6ft2)(Φ16", 12cells) 3.9m² (42ft2)(Φ16", 15cells) 4.16m² (44.8ft2)(Φ16", 16cells) |
| Material of Constructions | |
| Media | Cellulose/Diatomaceous earth/Perlite/Resins, etc. |
| Support/Diversion | Polypropylene |
| Seal Material | Silicone, EPDM, NBR, FKM |
| Performance | |
| Max. Operating Temperature | 80° C (176°F) |
| Max. Operating DP | 2Bar(29psi)@25℃(77℉) 1Bar(14.5psi)@80℃(176℉) |