Bioaxenic-A Capsule Filters
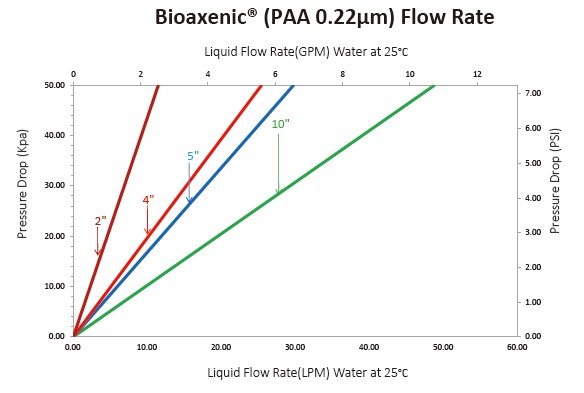
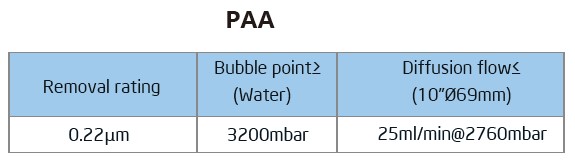
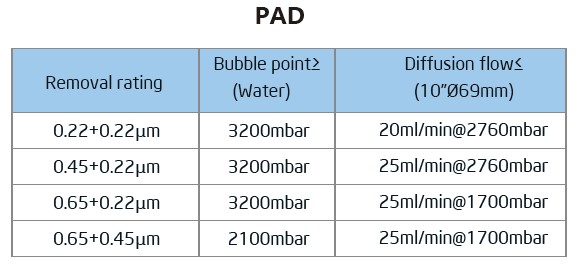
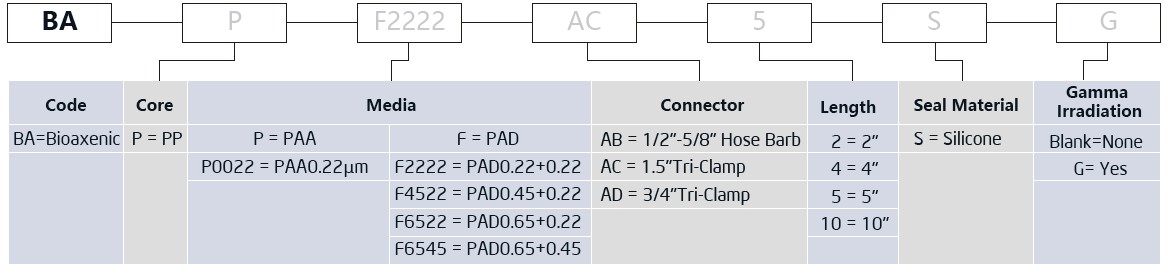
The Bioaxenic®-A Capsule Filters are researched and devel-oped for sterile filtration process with reliable and stable sterilization performance. It’s designed with external vent valve structure that can remove Gas-liquid quick...
The Bioaxenic®-A Capsule Filters are researched and devel-oped for sterile filtration process with reliable and stable sterilization performance. It’s designed with external vent valve structure that can remove Gas-liquid quickly and efficiently. Its built-in sterile grade hydrophilic polyethersul-fone (PES) membrane has the characteristics of high flow rate, high contaminant holding capacity and excellent chemical compatibility, ensuring its wide range of applications such as bioburden and bacteria removal in the culture medium, intermediate solution, buffers and other upstream and down-stream processes. The Bioaxenic®-A Capsule Filters can help shorten process time, reduce production costs and improve economic benefits.
| Dimension | |
| Outer Diameter | 87mm |
| Length / Filtration area | 2"(65mm) / 0.12m² 4"(103mm) / 0.22m² 5"(118mm) / 0.26m² 10"(266mm) / 0.65m² |
| Material of Constructions | |
| Media | PES |
| Support | PP |
| Cage/Core/End cap | PP |
| Seal Material Options | Silicone |
| Performance | |
| Max. Operating Temperature | 50 ℃(122℉) |
| Max. Operating DP | 5 Bar(73psi)@20℃(68℉) 3 Bar(44psi)@50℃(122℉) |
| Bio-safety | |
| Endotoxin | Comply with USP<85>, endotoxin content <0.25EU/ mL |
| Biocompatibility | Comply with USP<87>USP<88> |
| Fiber release | Comply with 21CFR210.3(b)(6) on "Non fiber" release regulations |
| Bacteria Retention | Validated with Brevundimonas diminuta (ATCC 19146) at107/cm² (0.22μm) |
| Autoclaving | 125℃, 30min, 3 Cycles |
| Gamma irradiation | 35 kGy |